Abstract
Background
The COVID-19 pandemic impacted cancer care in the U.S. in many ways. Changes in the frequency of laboratory assessments may lead to surveillance bias in real-world studies of progression in patients (pts) with MM. This study compared patterns of myeloma-protein (M-Protein) and free light chain (FLC) laboratory assessments and real-world endpoints in the one-year periods before and after the onset of the COVID-19 pandemic, overall and by race.
Methods
In this retrospective observational study, we used the nationwide de-identified, electronic health record-derived Flatiron Health database. The analysis included pts with MM who initiated first-line (1L) systemic therapy in the pre-pandemic (Mar 1 to Nov 30, 2019) or pandemic (Mar 1 to Nov 30, 2020) eligibility periods with follow-up ending on Mar 1, 2020 and Mar 1, 2021, respectively. The monthly rates of M-Protein and FLC labs were calculated as the number of assessments divided by the follow-up time during 1L in months. Real-world derived progression (dP) events were based on changes in abstracted M-Protein values or structured FLC labs and thresholds aligned to the International Myeloma Working Group (IMWG) criteria. Real-world overall survival (rwOS) and derived progression-free survival (dPFS) were followed from 1L initiation until the first of death, dP, or end of the study period using Kaplan-Meier methods. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) comparing survival outcomes between the pre-pandemic and pandemic periods with adjustment for age, gender, race, and International Staging System (ISS) Stage. Analyses of monthly assessment rates and survival endpoints were further stratified by race.
Results
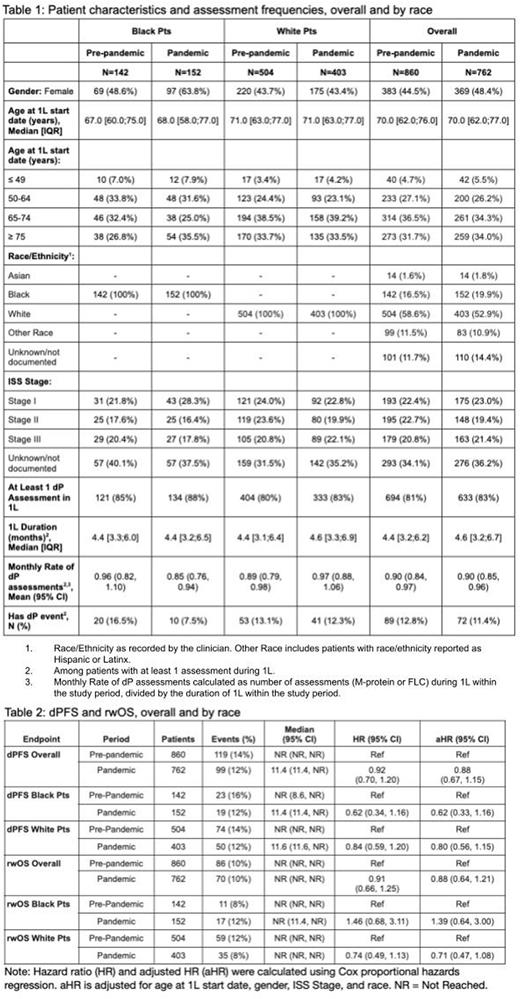
Overall, 860 (Black: 142; White: 504) eligible pts with MM were included in the pre-pandemic period and 762 (Black: 152; White: 403) in the pandemic period. Median dPFS and rwOS were not reached. Mean rates of assessments per month during 1L were approximately the same in the two periods (0.90 [95% CI 0.84-0.97] vs. 0.90 [95% CI 0.85-0.96]) overall, slightly lower in the pandemic period for Black pts (0.96 [95% CI 0.82-1.10] vs. 0.85 [95% CI 0.76-0.94]), and slightly higher in the pandemic period for White pts (0.89 [95% CI 0.79-0.98] vs. 0.97 [95% CI 0.88, 1.06]).
The proportion of pts initiating 1L in the pandemic period with an observed dP event was lower compared to the pre-pandemic period (12.8% [95% CI 10.5%-15.5%] vs. 11.4% [95% CI 9.1%-14.1%]), overall, and by race (Black: 16.5% [95% CI 11.0%-24.2%] vs 7.5% [95% CI 4.1%-13.2%]; White: 13.1% [95% CI 10.2%-16.8%] vs. 12.3% [95% CI 9.2%-16.3%]). In multivariable models, dPFS was similar comparing the pandemic period to pre-pandemic period (HR 0.88 95% CI 0.67, 1.15) overall and stratified by race (Black: HR 0.62, 95% CI 0.33-1.16; White: HR 0.80, 95% CI 0.56-1.15). The findings were consistent in multivariable analyses of rwOS comparing the pandemic period to the pre-pandemic period overall (HR 0.88, 95% CI 0.64-1.21), and by race (Black: HR 1.39, 95% CI 0.64-3.00; White: HR 0.71, 95% CI 0.47-1.08).
Conclusions
In this study of newly-diagnosed MM pts initiating 1L treatment prior to and during the COVID-19 pandemic, we did not observe statistically significant differences in rates of assessment for MM progression or survival outcomes when comparing the pre-pandemic and pandemic periods. The mean rate of assessments in the pandemic period was directionally lower in Black pts and higher in White pts, and the proportion of pts with a dP event was lower in the pandemic period for both Black and White pts, with the decrease being greater in Black pts. These findings do not support the hypothesis that COVID-19-related cancer care changes led to substantial surveillance bias in real-world studies of pts with MM. However, the results of analyses stratified by race suggest trends in racial inequities in cancer care during the COVID-19 pandemic that deserve clinical attention and further investigation.
Disclosures
Roose:Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Lu:Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Pierre:BMS: Consultancy; Roche: Current equity holder in publicly-traded company; Flatiron Health, Inc.: Current Employment; Pfizer: Consultancy. Sawas:Acrotech Biopharma: Consultancy, Speakers Bureau; Seagen: Consultancy, Speakers Bureau; Affimed: Research Funding; Roche: Current equity holder in publicly-traded company; Flatiron Health, Inc.: Current Employment; Daiichi Sankyo: Speakers Bureau. Calip:Pfizer: Research Funding; Roche: Current equity holder in publicly-traded company; Flatiron Health, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal